Hybrid Orbitals and Complex Molecules
In trying to understand the electronic structure of more complex molecules, it is always possible to proceed using the atomic eigenfunctions described previously as the basis states. However, it often makes more sense to think in terms of combinations of orbitals which have a maximum in electron density "pointing" at the atom with which the bond is to be made.
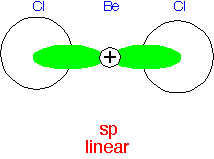
The combination of ⟨s| + ⟨p| makes an orbital that "points" in one direction, with ⟨s| - ⟨p| pointing in the opposite direction. Thus a linear molecule's bonds are likely to be close to "sp" hybrids.
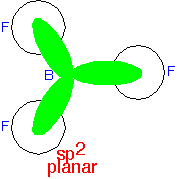
Three planar states at 120° angles can be constructed from two p orbitals and an s orbital, and used to describe a planar molecule: an sp2 state. The orbitals are (ignoring normalization) ⟨s| + ⟨px| + ⟨py|, ⟨s| + ⟨px| - ⟨py| and ⟨s| - ⟨px| - ⟨py|. In this case, one p orbital (typically we'd say pz in the example shown here) is not used in the hybrids and can be regarded as not participating in the bond.
All three p states and the s state can be combined into four orbitals pointing to the corners of a tetrahedron. These orbitals, the sp3 orbitals, are optimal for describing a molecule with 4 atoms coordinated to a central atom, allowing the coordinating atoms to adopt positions where they are all equal and as distant as possible from each other, minimizing any repulsive energy. The orbitals are
1/2 (s + px + py + pz)
1/2 (s - px - py - pz)
1/2 (s + px - py - pz)
1/2 (s - px + py - pz)
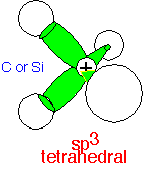
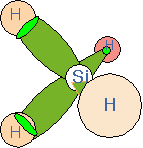
Let's consider an example of relevance to semiconductor deposition processes: the silane molecule, SiH4. Four hydrogen atoms are located tetrahedrally around the central silicon atom. We can think of the bonds as each being constructed from one sp3 hybrid on the silicon and the 1s atomic orbital on the hydrogen. All four bonds are identical and the bond-to-bond angles are all about 109 degrees.
If we were to remove a single hydrogen atom to produce SiH3, we would expect the molecule to rearrange itself into a planar configuration, with the bonds formed by sp2 orbitals from px and py, leaving the pz orbital in a "non-bonding" configuration perpendicular to the plane of the hydrogens. The pz orbital would be occupied by a single electron and therefore half-filled, able to accept another electron and form a bond. Such a reactive molecule, with an unpaired electron in an exposed orbital, is known as a radical.
On the other hand, a molecule of ammonia, NH3, has one additional electron relative to SiH3. Three of the outer shell electrons participate in covalent bonds with three hydrogen atoms; the two remaining electrons, rather than being in a pz-like orbital as in SiH4, occupy the fourth sp3-like orbital.
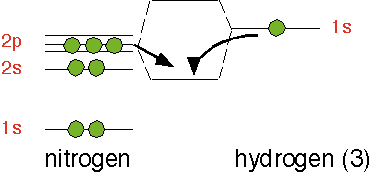
The electron state influences the geometry; rather than adopting a planar configuration as SiH3, ammonia is pyramidal: the three hydrogens are about at the corners of a tetrahedron, with the two additional electrons at the fourth corner in an sp3-like orbital that doesn't bond to another atom: they are a lone pair of electrons. This arrangement maximizes the distance between the bonds and thus minimizes the electrostatic repulsion of the electrons.
The lone pair electrons can readily interact with an atom with an empty orbital, such as the hydrogen ion H+ , or an electron-deficient neutral molecule such as BF3. The lone pair inserts itself into the empty orbital to form an adduct bond. The molecule with an electron pair to donate (NH3) is acting as a BASE, and the electron pair acceptor (H+) as an ACID.
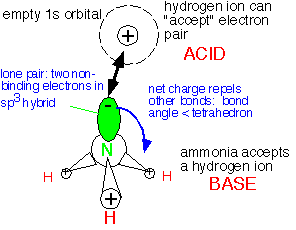
Note that the choice of proper basis functions -- that is, the appropriate way of thinking about the electronic structure of a molecule -- is strongly influenced by the geometry of the molecule. The behavior of a molecule is not merely determined by which atoms are in it, but also very much by where they are in relation to each other.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
