Mechanisms of Heat Transfer
Heat transfer occurs by three primary routes:
Conduction: the motion of heat through a stationary solid, liquid, or gas. Conduction in a gas takes place by the same mechanisms as mass transport. Heat conduction in a solid can be thought of as the diffusion of phonons (lattice vibrations). Conductive heat transfer can be described by the same formalism (the diffusion equation) that we used to study mass diffusion. However, in heat transport we'll find that even steady-state solutions are interesting, since fluxes generaly don't go to zero at surfaces as in the case of mass transport.
Convection: the physical transfer of gases or liquids containing heat energy. The simplistic treatment of convection we employed in studying mass transport is also useful; again we will find that we can often approximate transport in complex flows by assuming that diffusion takes place across streamlines and convection dominates along them.
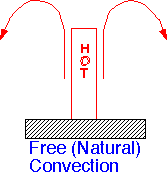
Natural Convection: Convective heat transport also introduces a new element: coupling of the fluid motion to the energy transfer. Hot gases are lighter than cool gases, and tend to rise in the presence of gravity. If temperature gradients are large enough, and externally imposed fluid velocities small enough, the expansion leads to convective transport driven by the heat flow: natural convection.
Radiation: Heat may be transferred even when there is nothing between two objects, by the motion of photons: radiative heat transfer. Radiative mechanisms often dominate in vacuum (but be careful! a few Torr of gas is enough to make conduction important in many cases).

Return to Tutorial Table of Contents
Book version of the CVD Tutorial
